What is the incidence of intracerebral hemorrhage (ICH) in the United States?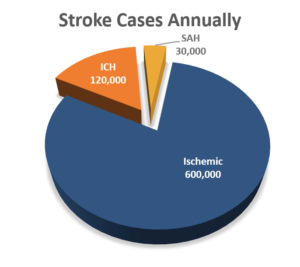
Intracerebral hemorrhage (ICH) is the second most common subtype of stroke and a critical disease usually leading to severe disability or death. In the US alone, there are 750,000 total stroke cases annually. 15% of all strokes are ICH.1 These are the deadliest subtype of stroke with a rate of mortality (within 30 days) = 30 – 50%.3
Over the last 10 years, hospital admissions for ICH have been up by 18% and there has been a high rate of disability in patients.4
ICH: It’s Deadly. Disability Is Likely. And It’s Costly.
![]()
Deadliest Form of Stroke
Rate of Mortality = 30 – 50%3
(within 30 days)
![]()
High Rate of Disability
Functional Independence
in ≈ 20% of patients4
![]() Costliest Form of Stroke5
Costliest Form of Stroke5
Ave Length of ICU Stay
= 11.9 Days
Cost per Day in ICU
= $2,007
Total Ave Cost per
Patient = $23,883
![]()
Increase in Hospital Admissions
Hospital admissions for ICH
are up by 18% over
the past 10 years
What challenges have been encountered with conventional surgical approaches?
- The need for clot stability has inhibited early intervention.6
- Concern for subcortical injury resulting from surgical access.7
- Failure to maximize clot reduction.8
- Inability to manage hemostasis.
- High rates of re-bleeding.10
How does the MIPS approach with NICO’s integrated systems solution overcome these challenges?
- Direct visualization
- Air medium
- Bi-manual technique for instrument convergence at site
- Hemostasis control
![]() Time is Brain
Time is Brain
Early intervention may mitigate:
- Hematoma expansion, edema & pressure
- Blood on brain toxicity
Solution: BrainPath enables navigable, trans-sulcal and parafascicular access.
- Minimally disruptive access with BrainPath that integrates advanced imaging with navigation.
Solution: Utilize Myriad NOVUS as your SUCKER and switch to CUTTER as needed.
- Minimize traction, and protect vasculature and normal parenchyma.
- Layer by layer resection of clot in-situ, utilizing very low levels of suction.
- Direct vision of clot and aperture interface.
- Single-point directionality.
![]()
Solution: Full ability to manage hemostasis through an air medium with direct visualization and bi-manual technique.
Similar to an open procedure, the BrainPath enables full use of micro-surgical technique to address the bleeding vessel directly through –
- Direct visualization
- Air medium
- Bi-manual technique for instrument convergence at site
- Use of Tisseal, cotton balls, patties, etc
NICO Myriad NOVUS = Ideal Tool for Removal & Collection
The NICO Myriad NOVUS handpiece delivers no thermal or ultrasonic energy avoiding heat related damage to healthy brain tissue.


Enrich Trial Summary
ENRICH is a multicenter, randomized, adaptive clinical trial comparing standard medical management to early surgical hematoma evacuation (less than 24 hours) using minimally invasive parafascicular surgery (MIPS) in the treatment of intracerebral hemorrhage (ICH), the deadliest, most costly and debilitating form of stroke worldwide. The acronym ENRICH means: Early MiNimally-invasive Removal of ICH.
The Emory Stroke Center of Emory University hospitals and the Marcus Stroke & Neuroscience Center of Grady Memorial Hospital leads the trial and it is sponsored by NICO Corporaton. The ENRICH trial compares the outcomes between early surgical intervention using the BrainPath® Approach (i.e., MIPS) and a medically managed cohort. The integrated surgical approach includes a combination of available technologies, including the FDA-cleared NICO BrainPath® for non-disruptive access and NICO Myriad NOVUS® to achieve the goal of maximum clot evacuation all through a smaller opening than traditional surgery. The medically managed cohort will be treated according the Clinical Standardization Guidelines (CSG) as adapted by Emory University from the 2015 AHA/ASA Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. Clinical efficacy will be determined by demonstrating a 10% improvement in functional outcome, as determined by a blinded-assessment of the 180-day utility-weighted modified Rankin Scale (mRS).
ICH is a major cause of morbidity and mortality and treatment of this disease remains a challenge for physicians, but improvement in critical care medicine, imaging technology, and surgical techniques have modestly improved patient survival. Data suggests improved mortality rates and potential functional benefits of surgical ICH evacuation. The methodology proposed for this trial was tested in a preliminary series of 39 patients treated for supratentorial spontaneous ICH and retrospectively reviewed (Labib et al.). These results were replicated in a single center retrospective series of 18 patients (Bain et al.). Despite positive results of both studies and the widely accepted benefit of the BrainPath Approach (i.e., MIPS) for subcortical lesions, stronger evidence supporting the use of these techniques in ICH is needed for the technique to become universally validated. ENRICH aims to contribute to the advancement and understanding of intracerebral hemorrhage care.
For more information visit the ENRICH Trial website at https://www.enrichtrial.com/
Key Publications
Citations
- Feigin VL, Lawes CM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8(4):355–69
- Ikram, et al. International Epidemiology of Intracerebral Hemorrhage. Curr Atheroscler Rep. 2012, Aug; 14(4): 300-306
- Stroke, 2014; 45:903-908
- Bilotta,et al. Neurocritical care for intracranial haemorrhage: a systematic review of recent studies. British Journal of Anaestesia. Volume 115, Issue suppl 2:Pii68-ii74
- Shah,et al. Technology that achieves the Triple Aim. ClinicoEconomics and Outcomes Research. 2017;9 519-523
- Sujijantarat, et al. “Trans-sulcal endoport assisted evacuation of supratentorial Intracerebral hemorrhage “Operative Neurosurgery 0:1-8,2017
- Kassam, et al “Part II: an evaluation of an integrated systems approach using diffusion weighted image guided exoscopic assisted transulcal radial corridors” Innovative Neurosurgery 2015
- Hanley, MISTIE II, International Stroke Conference, 2013
- Labib, et al, “The safety and feasibility of image-guided BrainPath mediated transsulcul hematoma evacuation: a Multicenter trial” Neurosurgery 4, April 2017: 515-524
- Bauer, et al.”Initial Single-center technical experience with the BrainPath system for acute Intracerebral hemorrhage evacuation” Operative Neurosurgery 2016
- Sujijantarat, et al. “Trans-sulcal endoport assisted evacuation of supratentorial Intracerebral hemorrhage “Operative Neurosurgery 0:1-8,2017
- Shah,et al. Technology that achieves the Triple Aim. ClinicoEconomics and Outcomes Research. 2017;9 519-523